BlossoMS Pregnancy Registry
What is the BlossoMS Pregnancy Registry?
The BlossoMS Pregnancy Registry was created to collect information about the possible effects of Vumerity® (diroximel fumarate), on pregnancy, delivery, and the health of babies. This information will be compared with data from women who have not had any MS therapy while pregnant.
The registry is looking for volunteers with multiple sclerosis (MS) who were prescribed diroximel fumarate by their doctor, and became pregnant while taking it. It is also looking for volunteers who have not had any MS therapy while pregnant. By contributing your information, you could help doctors to better understand the possible effects of diroximel fumarate on pregnancy, delivery, and the health of infants.
Participants will be followed throughout their pregnancy and information on the health of participants’ babies will also be collected until the baby is 12 months old.
By joining this registry and contributing your information, you may help women and their doctors make more informed decisions about medication use during pregnancy in the future. It is not known whether diroximel fumarate will harm unborn babies.
How do I participate?
Fill out the contact form to have a registry representative contact you with more information about this pregnancy registry
Call the registry's toll-free number
1-(833)-569-2635 and speak to a registry representative
Ask your healthcare provider to help you complete the contact form
Can I take part in this registry?
You may be able to take part in this registry if you are pregnant, and:
- have been diagnosed with multiple sclerosis (MS), and
- have taken Vumerity® (diroximel fumarate), at any time from 2 weeks after the first day of your last menstrual period through pregnancy, or
- have not had any MS therapy at any time during this pregnancy.
What will I have to do?
To take part in this pregnancy registry, you will first be asked to give verbal informed consent during a telephone interview with a registry representative. This means that you will receive information about the registry and what it will involve, have a chance to ask any questions, and decide if you want to take part.
If you decide to participate and verbally give your informed consent, then a registry representative will:
- Ask questions to collect some information about you and your pregnancy via telephone.
- Call you once each trimester to ask if there have been any changes in your pregnancy, your general health, or the medicines you are taking.
- Contact your and your baby’s healthcare providers during your pregnancy and after your delivery to ask them questions about your pregnancy, and your and your baby’s health. You may be contacted if your or your baby’s healthcare provider is unable to be reached.
If you would like to have further information to discuss with a family member or your regular doctor, or to share with others, you can download a copy of the BlossoMS Pregnancy Registry Patient Brochure.
Why and when will my healthcare providers be contacted?
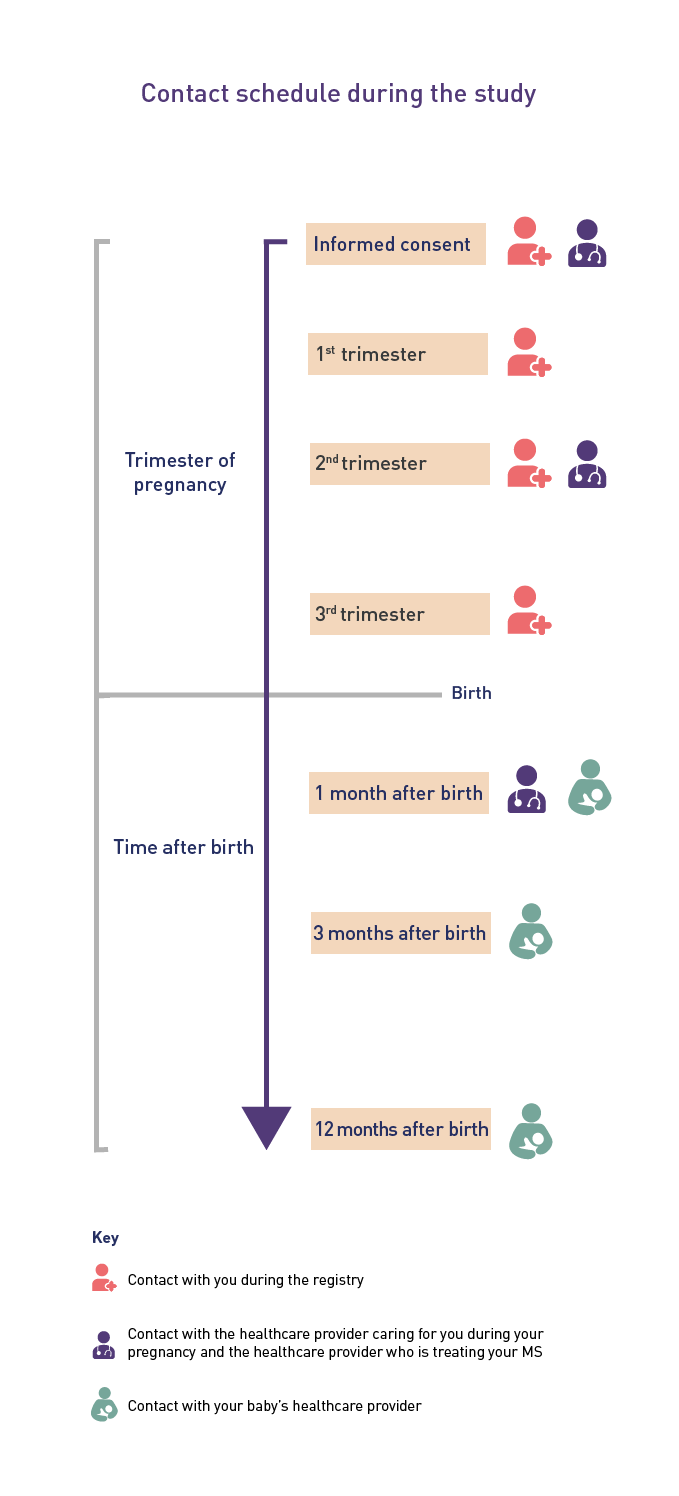
With your permission, a registry representative will contact the healthcare provider caring for you during your pregnancy, the healthcare provider who is treating your MS (if different than your regular obstetrician), and your baby’s healthcare provider. They will collect information about your general health, your pregnancy, and your baby’s health. Following your enrollment, a registry representative will:
- Contact your healthcare providers when you enroll in the registry, once during your second trimester, and 4 weeks after your due date, to ask about any changes in your pregnancy, general health, and treatment since the last call
- Contact your baby’s healthcare provider when your baby is about 1, 3, and 12 months old to ask about the health of your baby.
The contact you and your healthcare providers will receive is summarized in the diagram below.
If you participate in the registry, you will not:
- have any registry visits
- have any medical tests for the registry
- receive any medications for the registry.
What else will happen if I participate?
After you enroll in the registry, we will send you a welcome message by email. With your consent, we will also send you occasional emails and text messages to remind you about upcoming telephone interviews. You can choose to stop receiving these communications at any time. This will not affect your participation in the registry.
Our Privacy Policy contains full details of our commitment to your privacy.
Our FAQs contain answers to many frequently asked questions.
For the latest Important Safety Information, please refer to the full Prescribing Information and Medication Guide. This is not intended to replace discussions with your healthcare provider.